BCI Kickstarter #05 : Signal Processing in Python: Shaping EEG Data for BCI Applications
Welcome back to our BCI crash course! We've covered the fundamentals of BCIs, explored the brain's electrical activity, and equipped ourselves with the essential Python libraries for BCI development. Now, it's time to roll up our sleeves and dive into the practical world of signal processing. In this blog, we will transform raw EEG data into a format primed for BCI applications using MNE-Python. We will implement basic filters, create epochs around events, explore time-frequency representations, and learn techniques for removing artifacts. To make this a hands-on experience, we will work with the MNE sample dataset, a combined EEG and MEG recording from an auditory and visual experiment.

Getting Ready to Process: Load the Sample Dataset
First, let's load the sample dataset. If you haven't already, make sure you have MNE-Python installed (using conda install -c conda-forge mne). Then, run the following code:
import mne
# Load the sample dataset
data_path = mne.datasets.sample.data_path()
raw_fname = data_path + '/MEG/sample/sample_audvis_filt-0-40_raw.fif'
raw = mne.io.read_raw_fif(raw_fname, preload=True)
# Set the EEG reference to the average
raw.set_eeg_reference('average')
This code snippet loads the EEG data from the sample dataset into a raw object, ready for our signal processing adventures.
Implementing Basic Filters: Refining the EEG Signal
Raw EEG data is often contaminated by noise and artifacts from various sources, obscuring the true brain signals we're interested in. Filtering is a fundamental signal processing technique that allows us to selectively remove unwanted frequencies from our EEG signal.
Applying Filters with MNE: Sculpting the Frequency Landscape
MNE-Python provides a simple yet powerful interface for applying different types of filters to our EEG data using the raw.filter() function. Let's explore the most common filter types:
- High-Pass Filtering: Removes slow drifts and DC offsets, often caused by electrode movement or skin potentials. These low-frequency components can distort our analysis and make it difficult to identify event-related brain activity. Apply a high-pass filter with a cutoff frequency of 0.1 Hz to our sample data using:
raw_highpass = raw.copy().filter(l_freq=0.1, h_freq=None)
- Low-Pass Filtering: Removes high-frequency noise, which can originate from muscle activity or electrical interference. This noise can obscure the slower brain rhythms we're often interested in, such as alpha or beta waves. Apply a low-pass filter with a cutoff frequency of 30 Hz using:
raw_lowpass = raw.copy().filter(l_freq=None, h_freq=30)
- Band-Pass Filtering: Combines high-pass and low-pass filtering to isolate a specific frequency band. This is useful when we're interested in analyzing activity within a particular frequency range, such as the alpha band (8-12 Hz), which is associated with relaxed wakefulness. Apply a band-pass filter to isolate the alpha band using:
raw_bandpass = raw.copy().filter(l_freq=8, h_freq=12)
- Notch Filtering: Removes a narrow band of frequencies, typically used to eliminate power line noise (50/60 Hz) or other specific interference. This noise can create rhythmic artifacts in our data that can interfere with our analysis. Apply a notch filter at 50 Hz using:
raw_notch = raw.copy().notch_filter(freqs=50)
Visualizing Filtered Data: Observing the Effects
To see how filtering shapes our EEG signal, let's visualize the results using MNE-Python's plotting functions:
- Time-Domain Plots: Plot the raw and filtered EEG traces in the time domain using raw.plot(), raw_highpass.plot(), etc. Observe how the different filters affect the appearance of the signal.
- PSD Plots: Visualize the power spectral density (PSD) of the raw and filtered data using raw.plot_psd(), raw_highpass.plot_psd(), etc. Notice how filtering modifies the frequency content of the signal, attenuating power in the filtered bands.
Experiment and Explore: Shaping Your EEG Soundscape
Now it's your turn! Experiment with applying different filter settings to the sample dataset. Change the cutoff frequencies, try different filter types, and observe how the resulting EEG signal is transformed. This hands-on exploration will give you a better understanding of how filtering can be used to refine EEG data for BCI applications.
Epoching and Averaging: Extracting Event-Related Brain Activity
Filtering helps us refine the overall EEG signal, but for many BCI applications, we're interested in how the brain responds to specific events, such as the presentation of a stimulus or a user action. Epoching and averaging are powerful techniques that allow us to isolate and analyze event-related brain activity.
What are Epochs? Time-Locked Windows into Brain Activity
An epoch is a time-locked segment of EEG data centered around a specific event. By extracting epochs, we can focus our analysis on the brain's response to that event, effectively separating it from ongoing background activity.
Finding Events: Marking Moments of Interest
The sample dataset includes dedicated event markers, indicating the precise timing of each stimulus presentation and button press. We can extract these events using the mne.find_events() function:
events = mne.find_events(raw, stim_channel='STI 014')
This code snippet identifies the event markers from the STI 014 channel, commonly used for storing event information in EEG recordings.
Creating Epochs with MNE: Isolating Event-Related Activity
Now, let's create epochs around the events using the mne.Epochs() function:
# Define event IDs for the auditory stimuli
event_id = {'left/auditory': 1, 'right/auditory': 2}
# Set the epoch time window
tmin = -0.2 # 200 ms before the stimulus
tmax = 0.5 # 500 ms after the stimulus
# Create epochs
epochs = mne.Epochs(raw, events, event_id, tmin, tmax, baseline=(-0.2, 0))
This code creates epochs for the left and right auditory stimuli, spanning a time window from 200 ms before to 500 ms after each stimulus onset. The baseline argument applies baseline correction, subtracting the average activity during the pre-stimulus period (-200 ms to 0 ms) to remove any pre-existing bias.
Visualizing Epochs: Exploring Individual Responses
The epochs.plot() function allows us to explore individual epochs and visually inspect the data for artifacts:
epochs.plot()
This interactive visualization displays each epoch as a separate trace, allowing us to see how the EEG signal changes in response to the stimulus. We can scroll through epochs, zoom in on specific time windows, and identify any trials that contain excessive noise or artifacts.
Averaging Epochs: Revealing Event-Related Potentials
To reveal the consistent brain response to a specific event type, we can average the epochs for that event. This averaging process reduces random noise and highlights the event-related potential (ERP), a characteristic waveform reflecting the brain's processing of the event.
# Average the epochs for the left auditory stimulus
evoked_left = epochs['left/auditory'].average()
# Average the epochs for the right auditory stimulus
evoked_right = epochs['right/auditory'].average()
Plotting Evoked Responses: Visualizing the Average Brain Response
MNE-Python provides a convenient function for plotting the average evoked response:
evoked_left.plot()
evoked_right.plot()
This visualization displays the average ERP waveform for each auditory stimulus condition, showing how the brain's electrical activity changes over time in response to the sounds.
Analyze and Interpret: Unveiling the Brain's Auditory Processing
Now it's your turn! Analyze the evoked responses for the left and right auditory stimuli. Compare the waveforms, looking for differences in amplitude, latency, or morphology. Can you identify any characteristic ERP components, such as the N100 or P300? What do these differences tell you about how the brain processes sounds from different spatial locations?
Time-Frequency Analysis: Unveiling Dynamic Brain Rhythms
Epoching and averaging allow us to analyze the brain's response to events in the time domain. However, EEG signals are often non-stationary, meaning their frequency content changes over time. To capture these dynamic shifts in brain activity, we turn to time-frequency analysis.
Time-frequency analysis provides a powerful lens for understanding how brain rhythms evolve in response to events or cognitive tasks. It allows us to see not just when brain activity changes but also how the frequency content of the signal shifts over time.
Wavelet Transform with MNE: A Window into Time and Frequency
The wavelet transform is a versatile technique for time-frequency analysis. It decomposes the EEG signal into a set of wavelets, functions that vary in both frequency and time duration, providing a detailed representation of how different frequencies contribute to the signal over time.
MNE-Python offers the mne.time_frequency.tfr_morlet() function for computing the wavelet transform:
from mne.time_frequency import tfr_morlet
# Define the frequencies of interest
freqs = np.arange(7, 30, 1) # From 7 Hz to 30 Hz in 1 Hz steps
# Set the number of cycles for the wavelets
n_cycles = freqs / 2. # Increase the number of cycles with frequency
# Compute the wavelet transform for the left auditory epochs
power_left, itc_left = tfr_morlet(epochs['left/auditory'], freqs=freqs, n_cycles=n_cycles, use_fft=True, return_itc=True)
# Compute the wavelet transform for the right auditory epochs
power_right, itc_right = tfr_morlet(epochs['right/auditory'], freqs=freqs, n_cycles=n_cycles, use_fft=True, return_itc=True)
This code computes the wavelet transform for the left and right auditory epochs, focusing on frequencies from 7 Hz to 30 Hz. The n_cycles parameter determines the time resolution and frequency smoothing of the transform.
Visualizing Time-Frequency Representations: Spectrograms of Brain Activity
To visualize the time-frequency representations, we can use the mne.time_frequency.AverageTFR.plot() function:
power_left.plot([0], baseline=(-0.2, 0), mode='logratio', title="Left Auditory Stimulus")
power_right.plot([0], baseline=(-0.2, 0), mode='logratio', title="Right Auditory Stimulus")
This code displays spectrograms, plots that show the power distribution across frequencies over time. The baseline argument normalizes the power values to the pre-stimulus period, highlighting event-related changes.
Interpreting Time-Frequency Results
Time-frequency representations reveal how the brain's rhythmic activity evolves over time. Increased power in specific frequency bands after the stimulus can indicate the engagement of different cognitive processes. For example, we might observe increased alpha power during sensory processing or enhanced beta power during attentional engagement.
Discovering Dynamic Brain Patterns
Now, explore the time-frequency representations for the left and right auditory stimuli. Look for changes in power across different frequency bands following the stimulus onset. Do you observe any differences between the two conditions? What insights can you gain about the dynamic nature of auditory processing in the brain?
Artifact Removal Techniques: Cleaning Up Noisy Data
Even after careful preprocessing, EEG data can still contain artifacts that distort our analysis and hinder BCI performance. This section explores techniques for identifying and removing these unwanted signals, ensuring cleaner and more reliable data for our BCI applications.
Identifying Artifacts: Spotting the Unwanted Guests
- Visual Inspection: We can visually inspect raw EEG traces (raw.plot()) and epochs (epochs.plot()) to identify obvious artifacts, such as eye blinks, muscle activity, or electrode movement.
- Automated Methods: Algorithms can automatically detect specific artifact patterns based on their characteristic features, such as the high amplitude and slow frequency of eye blinks.
Rejecting Noisy Epochs: Discarding the Troublemakers
One approach to artifact removal is to simply discard noisy epochs. We can set rejection thresholds based on signal amplitude using the reject parameter in the mne.Epochs() function:
# Set rejection thresholds for EEG and EOG channels
reject = dict(eeg=150e-6) # Reject epochs with EEG activity exceeding 150 µV
# Create epochs with rejection criteria
epochs = mne.Epochs(raw, events, event_id, tmin, tmax, baseline=(-0.2, 0), reject=reject)
This code rejects epochs where the peak-to-peak amplitude of the EEG signal exceeds 150 µV, helping to eliminate trials contaminated by high-amplitude artifacts.
Independent Component Analysis (ICA): Unmixing the Signal Cocktail
Independent component analysis (ICA) is a powerful technique for separating independent sources of activity within EEG data. It assumes that the recorded EEG signal is a mixture of independent signals originating from different brain regions and artifact sources.
MNE-Python provides the mne.preprocessing.ICA() function for performing ICA:
from mne.preprocessing import ICA
# Create an ICA object
ica = ICA(n_components=20, random_state=97)
# Fit the ICA to the EEG data
ica.fit(raw)
We can then visualize the independent components using ica.plot_components() and identify components that correspond to artifacts based on their characteristic time courses and scalp topographies. Once identified, these artifact components can be removed from the data, leaving behind cleaner EEG signals.
Experiment and Explore: Finding the Right Cleaning Strategy
Artifact removal is an art as much as a science. Experiment with different artifact removal techniques and settings to find the best strategy for your specific dataset and BCI application. Visual inspection, rejection thresholds, and ICA can be combined to achieve optimal results.
Mastering the Art of Signal Processing
We've journeyed through the essential steps of signal processing in Python, transforming raw EEG data into a form ready for BCI applications. We've implemented basic filters, extracted epochs, explored time-frequency representations, and tackled artifact removal, building a powerful toolkit for shaping and refining brainwave data.
Remember, careful signal processing is the foundation for reliable and accurate BCI development. By mastering these techniques, you're well on your way to creating innovative applications that translate brain activity into action.
Resources and Further Reading
- MIT course on Signals and Systems: https://ocw.mit.edu/courses/res-6-007-signals-and-systems-spring-2011/
- Book: Smith, S. W. (2002). The scientist and engineer's guide to digital signal processing. California Technical Publishing.
From Processed Signals to Intelligent Algorithms: The Next Level
This concludes our deep dive into signal processing techniques using Python and MNE-Python. You've gained valuable hands-on experience in cleaning up, analyzing, and extracting meaningful information from EEG data, setting the stage for the next exciting phase of our BCI journey.
In the next post, we'll explore the world of machine learning for BCI, where we'll train algorithms to decode user intent, predict mental states, and control external devices directly from brain signals. Get ready to witness the magic of intelligent algorithms transforming processed brainwaves into real-world BCI applications!

Capturing a biosignal is only the beginning. The real challenge starts once those tiny electrical fluctuations from your brain, heart, or muscles are recorded. What do they mean? How do we clean, interpret, and translate them into something both the machine and eventually we can understand? In this blog, we move beyond sensors to the invisible layer of algorithms and analysis that turns raw biosignal data into insight. From filtering and feature extraction to machine learning and real-time interpretation, this is how your body’s electrical language becomes readable.
Every heartbeat, every blink, every neural spark produces a complex trace of electrical or mechanical activity. These traces known collectively as biosignals are the raw currency of human-body intelligence. But in their raw form they’re noisy, dynamic, and difficult to interpret.
The transformation from raw sensor output to interpreted understanding is what we call biosignal processing. It’s the foundation of modern neuro- and bio-technology, enabling everything from wearable health devices to brain-computer interfaces (BCIs).
The Journey: From Raw Signal to Insight
When a biosignal sensor records, it captures a continuous stream of data—voltage fluctuations (in EEG, ECG, EMG), optical intensity changes, or pressure variations.
But that stream is messy. It includes baseline drift, motion artefacts, impedance shifts as electrodes dry, physiological artefacts (eye blinks, swallowing, jaw tension), and environmental noise (mains hum, electromagnetic interference).
Processing converts this noise-ridden stream into usable information, brain rhythms, cardiac cycles, muscle commands, or stress patterns.
Stage 1: Pre-processing — Cleaning the Signal
Before we can make sense of the body’s signals, we must remove the noise.
- Filtering: Band-pass filters (typically 0.5–45 Hz for EEG) remove slow drift and high-frequency interference; notch filters suppress 50/60 Hz mains hum.
- Artifact removal: Independent Component Analysis (ICA) and regression remain the most common methods for removing eye-blink (EOG) and muscle (EMG) artefacts, though hybrid and deep learning–based techniques are becoming more popular for automated denoising.
- Segmentation / epoching: Continuous biosignals are divided into stable time segments—beat-based for ECG or fixed/event-locked windows for EEG (e.g., 250 ms–1 s)—to capture temporal and spectral features more reliably.
- Normalization & baseline correction: Normalization rescales signal amplitudes across channels or subjects, while baseline correction removes constant offsets or drift to align signals to a common reference.
Think of this stage as cleaning a lens: if you don’t remove the smudges, everything you see through it will be distorted.
Stage 2: Feature Extraction — Finding the Patterns
Once the signal is clean, we quantify its characteristics, features that encode physiological or cognitive states.
Physiological Grounding
- EEG: Arises from synchronized postsynaptic currents in cortical pyramidal neurons.
- EMG: Records summed action potentials from contracting muscle fibers.
- ECG: Reflects rhythmic depolarization of cardiac pacemaker (SA node) cells.
Time-domain Features
Mean, variance, RMS, and zero-crossing rate quantify signal amplitude and variability over time. In EMG, Mean Absolute Value (MAV) and Waveform Length (WL) reflect overall muscle activation and fatigue progression.
Frequency & Spectral Features
The power of each EEG band tends to vary systematically across mental states.

Time–Frequency & Non-Linear Features
Wavelet transforms or Empirical Mode Decomposition capture transient events. Entropy- and fractal-based measures reveal complexity, useful for fatigue or cognitive-load studies.
Spatial Features
For multi-channel EEG, spatial filters such as Common Spatial Patterns (CSP) isolate task-specific cortical sources.
Stage 3: Classification & Machine Learning — Teaching Machines to Read the Body
After feature extraction, machine-learning models map those features to outcomes: focused vs fatigued, gesture A vs gesture B, normal vs arrhythmic.
- Classical ML: SVM, LDA, Random Forest , effective for curated features.
- Deep Learning: CNNs, LSTMs, Graph CNNs , learn directly from raw or minimally processed data.
- Transfer Learning: Improves cross-subject performance by adapting pretrained networks.
- Edge Inference: Deploying compact models (TinyML, quantized CNNs) on embedded hardware to achieve < 10 ms latency.
This is where raw physiology becomes actionable intelligence.
Interpreting Results — Making Sense of the Numbers
A robust pipeline delivers meaning, not just data:
- Detecting stress or fatigue for adaptive feedback.
- Translating EEG patterns into commands for prosthetics or interfaces.
- Monitoring ECG spectral shifts to flag early arrhythmias.
- Quantifying EMG coordination for rehabilitation or athletic optimization.
Performance hinges on accuracy, latency, robustness, and interpretability, especially when outcomes influence safety-critical systems.
Challenges and Future Directions
Technical: Inter-subject variability, electrode drift, real-world noise, and limited labeled datasets still constrain accuracy.
Ethical / Explainability: As algorithms mediate more decisions, transparency and consent are non-negotiable.
Multimodal Fusion: Combining EEG + EMG + ECG data improves reliability but raises synchronization and power-processing challenges.
Edge AI & Context Awareness: The next frontier is continuous, low-latency interpretation that adapts to user state and environment in real time.
Final Thought
Capturing a biosignal is only half the story. What truly powers next-gen neurotech and human-aware systems is turning that signal into sense. From electrodes and photodiodes to filters and neural nets, each link in this chain brings us closer to devices that don’t just measure humans; they understand them.

Every thought, heartbeat, and muscle twitch leaves behind a signal, but how do we actually capture them? In this blog post, we explore the sensors that make biosignal measurement possible, from EEG and ECG electrodes to optical and biochemical interfaces, and what it takes to turn those signals into meaningful data.
When we think of sensors, we often imagine cameras, microphones, or temperature gauges. But some of the most fascinating sensors aren’t designed to measure the world, they’re designed to measure you.
These are biosignal sensors: tiny, precise, and increasingly powerful tools that decode the electrical whispers of your brain, heart, and muscles. They're the hidden layer enabling brain-computer interfaces, wearables, neurofeedback systems, and next-gen health diagnostics.
But how do they actually work? And what makes one sensor better than another?
Let’s break it down, from scalp to circuit board.
First, a Quick Recap: What Are Biosignals?
Biosignals are the body’s internal signals, electrical, optical, or chemical , that reflect brain activity, heart function, muscle movement, and more. If you’ve read our earlier post on biosignal types, you’ll know they’re the raw material for everything from brain-computer interfaces to biometric wearables.
In this blog, we shift focus to the devices and sensors that make it possible to detect these signals in the real world, and what it takes to do it well.
The Devices That Listen In: Biosignal Sensor Types
.webp)
A Closer Look: How These Sensors Work
1. EEG / ECG / EMG – Electrical Sensors
These measure voltage fluctuations at the skin surface, caused by underlying bioelectric activity.
It’s like trying to hear a whisper in a thunderstorm; brain and muscle signals are tiny, and will get buried under noise unless the electrodes make solid contact and the amplifier filters aggressively.
There are two key electrode types:
- Wet electrodes: Use conductive gel or Saline for better signal quality. Still the gold standard in labs.
- Dry electrodes: More practical for wearables but prone to motion artifacts and noise (due to higher electrode resistance).
Signal acquisition often involves differential recording and requires high common-mode rejection ratios (CMRR) to suppress environmental noise.
Fun Fact: Even blinking your eyes generates an EMG signal that can overwhelm EEG data. That’s why artifact rejection algorithms are critical in EEG-based systems.
2. Optical Sensors (PPG, fNIRS)
These use light to infer blood flow or oxygenation levels:
- PPG: Emits light into the skin and measures reflection, pulsatile blood flow alters absorption.
- fNIRS: Uses near-infrared light to differentiate oxygenated vs. deoxygenated hemoglobin in the cortex.
Example: Emerging wearable fNIRS systems like Kernel Flow and OpenBCI Galea are making brain oxygenation measurement accessible outside labs.
3. Galvanic Skin Response / EDA – Emotion’s Electrical Signature
GSR (also called electrodermal activity) sensors detect subtle changes in skin conductance caused by sweat gland activity, a direct output of sympathetic nervous system arousal. When you're stressed or emotionally engaged, your skin becomes more conductive, and GSR sensors pick that up.
These sensors apply a small voltage across two points on the skin and track resistance over time. They're widely used in emotion tracking, stress monitoring, and psychological research due to their simplicity and responsiveness.
Together, these sensors form the foundation of modern biosignal acquisition — but capturing clean signals isn’t just about what you use, it’s about how you use it.
How Signal Quality Is Preserved
Measurement is just step one; capturing clean, interpretable signals involves:
- Analog Front End (AFE): Amplifies low signals while rejecting noise.
- ADC: Converts continuous analog signals into digital data.
- Signal Conditioning: Filters out drift, DC offset, 50/60Hz noise.
- Artifact Removal: Eye blinks, jaw clenches, muscle twitches.
Hardware platforms like TI’s ADS1299 and Analog Devices’ MAX30003 are commonly used in EEG and ECG acquisition systems.
New Frontiers in Biosignal Measurement
- Textile Sensors: Smart clothing with embedded electrodes for long-term monitoring.
- Biochemical Sensors: Detect metabolites like lactate, glucose, or cortisol in sweat or saliva.
- Multimodal Systems: Combining EEG + EMG + IMU + PPG in unified setups to boost accuracy.
A recent study involving transradial amputees demonstrated that combining EEG and EMG signals via a transfer learning model increased classification accuracy by 2.5–4.3% compared to EEG-only models.
Other multimodal fusion approaches, such as combining EMG and force myography (FMG), have shown classification improvements of over 10% compared to EMG alone.
Why Should You Care?
Because how we measure determines what we understand, and what we can build.
Whether it's a mental wellness wearable, a prosthetic limb that responds to thought, or a personalized neurofeedback app, it all begins with signal integrity. Bad data means bad decisions. Good signals? They unlock new frontiers.
Final Thought
We’re entering an era where technology doesn’t just respond to clicks, it responds to cognition, physiology, and intent.
Biosignal sensors are the bridge. Understanding them isn’t just for engineers; it’s essential for anyone shaping the future of human-aware tech.

In our previous blog, we explored how biosignals serve as the body's internal language—electrical, mechanical, and chemical messages that allow us to understand and interface with our physiology. Among these, electrical biosignals are particularly important for understanding how our nervous system, muscles, and heart function in real time. In this article, we’ll take a closer look at three of the most widely used electrical biosignals—EEG, ECG, and EMG—and their growing role in neurotechnology, diagnostics, performance tracking, and human-computer interaction. If you're new to the concept of biosignals, you might want to check out our introductory blog for a foundational overview.
"The body is a machine, and we must understand its currents if we are to understand its functions."-Émil du Bois-Reymond, pioneer in electrophysiology.
Life, though rare in the universe, leaves behind unmistakable footprints—biosignals. These signals not only confirm the presence of life but also narrate what a living being is doing, feeling, or thinking. As technology advances, we are learning to listen to these whispers of biology. Whether it’s improving health, enhancing performance, or building Brain-Computer Interfaces (BCIs), understanding biosignals is key.
Among the most studied biosignals are:
- Electroencephalogram (EEG) – from the brain
- Electrocardiogram (ECG) – from the heart
- Electromyogram (EMG) – from muscles
- Galvanic Skin Response (GSR) – from skin conductance
These signals are foundational for biosignal processing, real-time monitoring, and interfacing the human body with machines. In this article we look at some of these biosignals and some fascinating stories behind them.
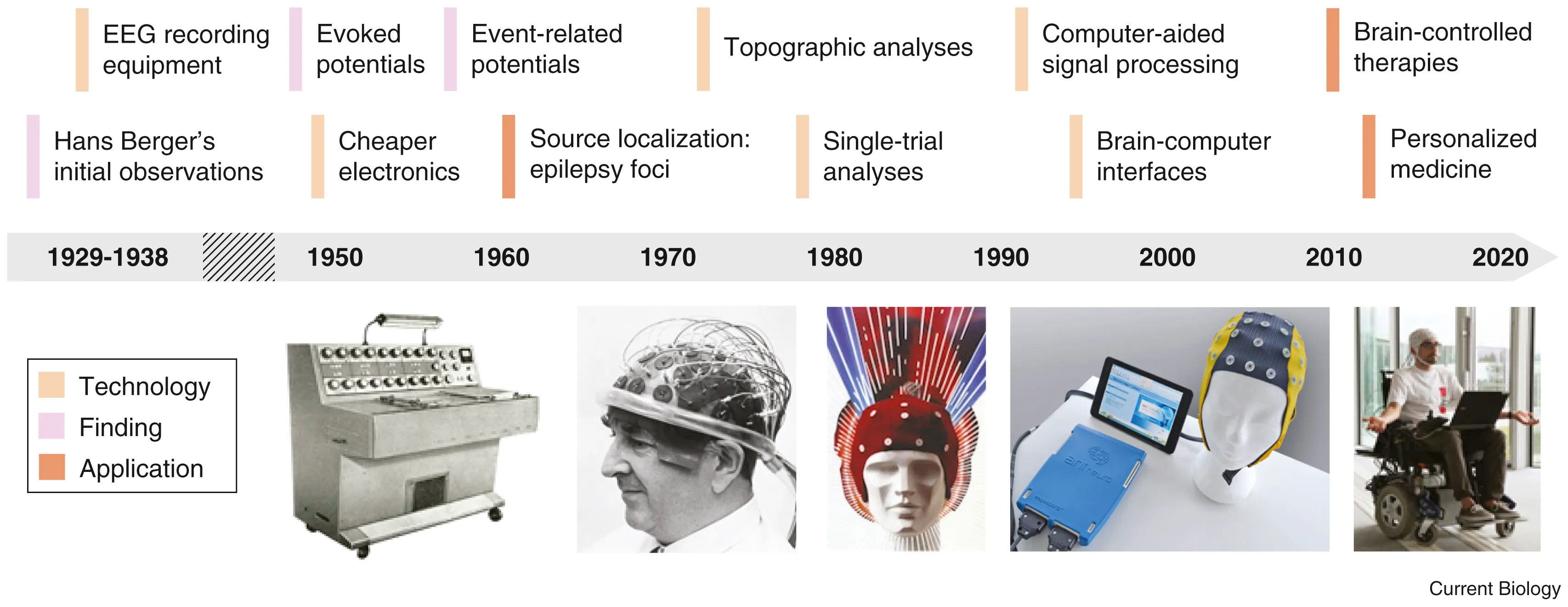
Electroencephalography (EEG): Listening to Brainwaves
In 1893, a 19 year old Hans Berger fell from a horse and had a near death experience. Little did he know that it would be a pivotal moment in the history of neurotechnology. The same day he received a telegram from his sister who was extremely concerned for him because she had a bad feeling. Hans Berger was convinced that this was due to the phenomenon of telepathy. After all, it was the age of radio waves, so why can’t there be “brain waves”? In his ensuing 30 year career telepathy was not established but in his pursuit, Berger became the first person to record brain waves.
When neurons fire together, they generate tiny electrical currents. These can be recorded using electrodes placed on the scalp (EEG), inside the skull (intracranial EEG), or directly on the brain (ElectroCorticogram). EEG signal processing is used not only to understand the brain’s rhythms but also in EEG-based BCI systems, allowing communication and control for people with paralysis. Event-Related Potentials (ERPs) and Local Field Potentials (LFPs) are specialized types of EEG signals that provide insights into how the brain responds to specific stimuli.
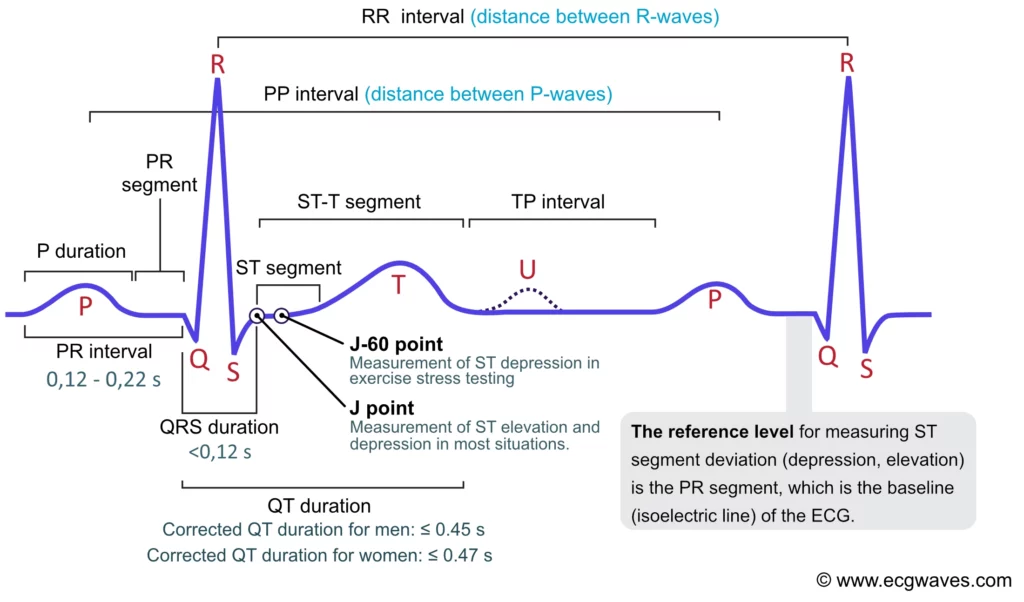
Electrocardiogram (ECG): The Rhythm of the Heart
The heart has its own internal clock which produces tiny electrical signals every time it beats. Each heartbeat starts with a small electrical impulse made by a special part of the heart called the sinoatrial (SA) node. This impulse spreads through the heart muscle and makes it contract, first the upper (atria) and then lower chambers (ventricles) – that’s what pumps blood. This process produces voltage changes, which can be recorded via electrodes on the skin.
This gives rise to the classic PQRST waveform, with each component representing a specific part of the heart’s cycle. Modern wearables and medical devices use ECG signal analysis to monitor heart health in real time.
Fun fact: The waveform starts with “P” because Willem Einthoven left room for earlier letters—just in case future scientists discovered pre-P waves! So, thanks to a cautious scientist, we have the quirky naming system we still follow today.
Electromyography (EMG): The Language of Movement
When we perform any kind of movement - lifting our arm, kicking our leg, smiling, blinking or even breathing- our brain sends electrical signals to our muscles telling them to contract. When these neurons, known as motor neurons fire they release electrical impulses that travel to the muscle, causing it to contract. This electrical impulse—called a motor unit action potential (MUAP)—is what we see as an EMG signal. So, every time we move, we are generating an EMG signal!
Medical Applications
Medically, EMG is used for monitoring muscle fatigue especially in rehabilitation settings and muscle recovery post-injury or surgery. This helps clinicians measure progress and optimize therapy. EMG can distinguish between voluntary and involuntary movements, making it useful in diagnosing neuromuscular disorders, assessing stroke recovery, spinal cord injuries, and motor control dysfunctions.
Performance and Sports Science
In sports science, EMG can tell us muscle-activation timing and quantify force output of muscle groups. These are important factors to measure performance improvement in any sport. The number of motor units recruited and the synergy between muscle groups, helps us capture “mind-muscle connection” and muscle memory. Such things which were previously spoken off in a figurative manner can be scientifically measured and quantified using EMG. By tracking these parameters we get a window into movement efficiency and athletic performance. EMG is also used for biofeedback training, enabling individuals to consciously correct poor movement habits or retrain specific muscles
Beyond medicine and sports, EMG is used for gesture recognition in AR/VR and gaming, silent speech detection via facial EMG, and next-gen prosthetics and wearable exosuits that respond to the user’s muscle signals. EMG can be used in brain-computer interfaces (BCIs), helping paralyzed individuals control digital devices or communicate through subtle muscle activity. EMG bridges the gap between physiology, behavior, and technology—making it a critical tool in healthcare, performance optimization, and human-machine interaction.
As biosignal processing becomes more refined and neurotech devices more accessible, we are moving toward a world where our body speaks—and machines understand. Whether it’s detecting the subtlest brainwaves, tracking a racing heart, or interpreting muscle commands, biosignals are becoming the foundation of the next digital revolution. One where technology doesn’t just respond, but understands.

