BCI Kickstarter #03: EEG Signal Acquisition and Processing
Welcome back to our BCI crash course! In the previous blog, we explored the basic concepts of BCIs and delved into the fundamentals of neuroscience. Now, it's time to get our hands dirty with the practical aspects of EEG signal acquisition and processing. This blog will guide you through the journey of transforming raw EEG data into a format suitable for meaningful analysis and BCI applications. We will cover signal preprocessing techniques, and feature extraction methods, providing you with the essential tools for decoding the brain's electrical secrets.

Signal Preprocessing Techniques: Cleaning Up the Data
Raw EEG data, fresh from the electrodes, is often a noisy and complex landscape. To extract meaningful insights and develop reliable BCIs, we need to apply various signal preprocessing techniques to clean up the data, remove artifacts, and enhance the true brain signals.
Why Preprocessing is Necessary: Navigating a Sea of Noise
The journey from raw EEG recordings to usable data is fraught with challenges:
- Noise and Artifacts Contamination: EEG signals are susceptible to various sources of interference, both biological (e.g., muscle activity, eye blinks, heartbeats) and environmental (e.g., power line noise, electrode movement). These artifacts can obscure the true brain signals we are interested in.
- Separating True Brain Signals: Even in the absence of obvious artifacts, raw EEG data contains a mix of neural activity related to various cognitive processes. Preprocessing helps us isolate the specific signals relevant to our research or BCI application.
Importing Data: Laying the Foundation
Before we can begin preprocessing, we need to import our EEG data into a suitable software environment. Common EEG data formats include:
- FIF (Functional Imaging File Format): A widely used format developed for MEG and EEG data, supported by the MNE library in Python.
- EDF (European Data Format): Another standard format, often used for clinical EEG recordings.
Libraries like MNE provide functions for reading and manipulating these formats, enabling us to work with EEG data in a programmatic way.
Removing Bad Channels and Interpolation: Dealing with Faulty Sensors
Sometimes, EEG recordings contain bad channels — electrodes that are malfunctioning, poorly placed, or picking up excessive noise. We need to identify and address these bad channels before proceeding with further analysis.
Identifying Bad Channels:
- Visual Inspection: Plotting the raw EEG data and visually identifying channels with unusually high noise levels, flat lines, or other anomalies.
- Automated Methods: Using algorithms that detect statistically significant deviations from expected signal characteristics.
Interpolation:
If a bad channel cannot be salvaged, we can use interpolation to estimate its missing data based on the surrounding good channels. Spherical spline interpolation is a common technique that projects electrode locations onto a sphere and uses a mathematical model to estimate the missing values.
Filtering: Tuning into the Right Frequencies
Filtering is a fundamental preprocessing step that allows us to remove unwanted frequencies from our EEG signal. Different types of filters serve distinct purposes:
- High-Pass Filtering: Removes slow drifts and DC offsets, which are often caused by electrode movement or skin potentials. A typical cutoff frequency for high-pass filtering is around 0.1 Hz.
- Low-Pass Filtering: Removes high-frequency noise, which can originate from muscle activity or electrical interference. A common cutoff frequency for low-pass filtering is around 30 Hz for most cognitive tasks, though some applications may use higher cutoffs for studying gamma activity.
- Band-Pass Filtering: Combines high-pass and low-pass filtering to isolate a specific frequency band of interest, such as the alpha (8-12 Hz) or beta (12-30 Hz) band.
- Notch Filtering: Removes a narrow band of frequencies, typically used to eliminate power line noise (50/60 Hz) or other specific interference.
Choosing the appropriate filter settings is crucial for isolating the relevant brain signals and minimizing the impact of noise on our analysis.
Downsampling: Reducing the Data Load
Downsampling refers to reducing the sampling rate of our EEG signal, which can be beneficial for:
- Reducing data storage requirements: Lower sampling rates result in smaller file sizes.
- Improving computational efficiency: Processing lower-resolution data requires less computing power.
However, we need to be cautious when downsampling to avoid losing important information. The Nyquist-Shannon sampling theorem dictates that we must sample at a rate at least twice the highest frequency of interest in our signal to avoid aliasing, where high frequencies are incorrectly represented as lower frequencies.
Decimation is a common downsampling technique that combines low-pass filtering with sample rate reduction to ensure that we don't introduce aliasing artifacts into our data.
Re-Referencing: Choosing Your Point of View
In EEG recording, each electrode's voltage is measured relative to a reference electrode. The choice of reference can significantly influence the interpretation of our signals, as it affects the baseline against which brain activity is measured.
Common reference choices include:
- Linked Mastoids: Averaging the signals from the mastoid electrodes behind each ear.
- Average Reference: Averaging the signals from all electrodes.
- Other References: Specific electrodes (e.g., Cz) or combinations of electrodes can be chosen based on the research question or BCI application.
Re-referencing allows us to change the reference of our EEG data after it's been recorded. This can be useful for comparing data recorded with different reference schemes or for exploring the impact of different references on signal interpretation. Libraries like MNE provide functions for easily re-referencing data.
Feature Extraction Methods: Finding the Signal in the Noise
Once we've preprocessed our EEG data, it's time to extract meaningful information that can be used for analysis or to train BCI systems. Feature extraction is the process of transforming the preprocessed EEG signal into a set of representative features that capture the essential patterns and characteristics of the underlying brain activity.
What is Feature Extraction? Simplifying the Data Landscape
Raw EEG data, even after preprocessing, is often high-dimensional and complex. Feature extraction serves several important purposes:
- Reducing Data Dimensionality: By extracting a smaller set of representative features, we simplify the data, making it more manageable for analysis and machine learning algorithms.
- Highlighting Relevant Patterns: Feature extraction methods focus on specific aspects of the EEG signal that are most relevant to the research question or BCI application, enhancing the signal-to-noise ratio and improving the accuracy of our analyses.
Time-Domain Features: Analyzing Signal Fluctuations
Time-domain features capture the temporal characteristics of the EEG signal, focusing on how the voltage changes over time. Some common time-domain features include:
- Amplitude:
- Peak-to-Peak Amplitude: The difference between the highest and lowest voltage values within a specific time window.
- Mean Amplitude: The average voltage value over a given time period.
- Variance: A measure of how much the signal fluctuates around its mean value.
- Peak-to-Peak Amplitude: The difference between the highest and lowest voltage values within a specific time window.
- Latency:
- Onset Latency: The time it takes for a specific event-related potential (ERP) component to appear after a stimulus.
- Peak Latency: The time point at which an ERP component reaches its maximum amplitude.
- Onset Latency: The time it takes for a specific event-related potential (ERP) component to appear after a stimulus.
- Time-Series Analysis:
- Autoregressive Models: Statistical models that predict future values of the signal based on its past values, capturing temporal dependencies in the data.
- Moving Averages: Smoothing techniques that calculate the average of the signal over a sliding window, reducing noise and highlighting trends.
- Autoregressive Models: Statistical models that predict future values of the signal based on its past values, capturing temporal dependencies in the data.
Frequency-Domain Features: Unveiling the Brain's Rhythms
Frequency-domain features analyze the EEG signal in the frequency domain, revealing the power distribution across different frequency bands. Key frequency-domain features include:
- Power Spectral Density (PSD): A measure of the signal's power at different frequencies. PSD is typically calculated using the Fast Fourier Transform (FFT), which decomposes the signal into its constituent frequencies.
- Band Power: The total power within a specific frequency band, such as delta, theta, alpha, beta, or gamma. Band power features are often used in BCI systems to decode mental states or user intent.
Time-Frequency Features: Bridging the Time and Frequency Divide
Time-frequency features provide a combined view of the EEG signal in both time and frequency domains, capturing dynamic changes in frequency content over time. Important time-frequency features include:
- Wavelet Transform: A powerful technique that decomposes the signal into a set of wavelets, functions that vary in both frequency and time duration. Wavelet transforms excel at capturing transient events and analyzing signals with non-stationary frequency content.
- Short-Time Fourier Transform (STFT): Divides the signal into short segments and calculates the FFT for each segment, providing a time-varying spectrum. STFT is useful for analyzing how the frequency content of the signal changes over time.
From Raw Signals to Actionable Insights
The journey from raw EEG data to meaningful insights and BCI control involves a carefully orchestrated sequence of signal acquisition, preprocessing, and feature extraction. Each step plays a crucial role in revealing the hidden patterns within the brain's electrical symphony, allowing us to decode mental states, control external devices, and unlock new possibilities for human-computer interaction.
By mastering these techniques, we can transform the complex and noisy world of EEG recordings into a rich source of information, paving the way for innovative BCI applications that can improve lives and expand our understanding of the human brain.
Further Reading and Resources
- Book: Analyzing Neural Time Series Data: Theory and Practice
By: Mike X Cohen
https://doi.org/10.7551/mitpress/9609.001.0001
ISBN (electronic): 9780262319553
- Tutorial: MNE-Python documentation on preprocessing: https://mne.tools/stable/auto_tutorials/preprocessing/index.html
- Article: Urigüen, J. A., & Garcia-Zapirain, B. (2015). EEG artifact removal—state-of-the-art and guidelines. Journal of Neural Engineering, 12(3), 031001.
What's Next: Real-World BCIs using Signal Processing
This concludes our exploration of EEG signal acquisition and processing. Now that we've learned how to clean up and extract meaningful features from raw EEG data, we are ready to explore how these techniques are used to build real-world BCI applications.
In the next post, we'll dive into the fascinating world of BCI paradigms and applications, discovering the diverse ways BCIs are being used to translate brain signals into actions. Stay tuned!

Capturing a biosignal is only the beginning. The real challenge starts once those tiny electrical fluctuations from your brain, heart, or muscles are recorded. What do they mean? How do we clean, interpret, and translate them into something both the machine and eventually we can understand? In this blog, we move beyond sensors to the invisible layer of algorithms and analysis that turns raw biosignal data into insight. From filtering and feature extraction to machine learning and real-time interpretation, this is how your body’s electrical language becomes readable.
Every heartbeat, every blink, every neural spark produces a complex trace of electrical or mechanical activity. These traces known collectively as biosignals are the raw currency of human-body intelligence. But in their raw form they’re noisy, dynamic, and difficult to interpret.
The transformation from raw sensor output to interpreted understanding is what we call biosignal processing. It’s the foundation of modern neuro- and bio-technology, enabling everything from wearable health devices to brain-computer interfaces (BCIs).
The Journey: From Raw Signal to Insight
When a biosignal sensor records, it captures a continuous stream of data—voltage fluctuations (in EEG, ECG, EMG), optical intensity changes, or pressure variations.
But that stream is messy. It includes baseline drift, motion artefacts, impedance shifts as electrodes dry, physiological artefacts (eye blinks, swallowing, jaw tension), and environmental noise (mains hum, electromagnetic interference).
Processing converts this noise-ridden stream into usable information, brain rhythms, cardiac cycles, muscle commands, or stress patterns.
Stage 1: Pre-processing — Cleaning the Signal
Before we can make sense of the body’s signals, we must remove the noise.
- Filtering: Band-pass filters (typically 0.5–45 Hz for EEG) remove slow drift and high-frequency interference; notch filters suppress 50/60 Hz mains hum.
- Artifact removal: Independent Component Analysis (ICA) and regression remain the most common methods for removing eye-blink (EOG) and muscle (EMG) artefacts, though hybrid and deep learning–based techniques are becoming more popular for automated denoising.
- Segmentation / epoching: Continuous biosignals are divided into stable time segments—beat-based for ECG or fixed/event-locked windows for EEG (e.g., 250 ms–1 s)—to capture temporal and spectral features more reliably.
- Normalization & baseline correction: Normalization rescales signal amplitudes across channels or subjects, while baseline correction removes constant offsets or drift to align signals to a common reference.
Think of this stage as cleaning a lens: if you don’t remove the smudges, everything you see through it will be distorted.
Stage 2: Feature Extraction — Finding the Patterns
Once the signal is clean, we quantify its characteristics, features that encode physiological or cognitive states.
Physiological Grounding
- EEG: Arises from synchronized postsynaptic currents in cortical pyramidal neurons.
- EMG: Records summed action potentials from contracting muscle fibers.
- ECG: Reflects rhythmic depolarization of cardiac pacemaker (SA node) cells.
Time-domain Features
Mean, variance, RMS, and zero-crossing rate quantify signal amplitude and variability over time. In EMG, Mean Absolute Value (MAV) and Waveform Length (WL) reflect overall muscle activation and fatigue progression.
Frequency & Spectral Features
The power of each EEG band tends to vary systematically across mental states.

Time–Frequency & Non-Linear Features
Wavelet transforms or Empirical Mode Decomposition capture transient events. Entropy- and fractal-based measures reveal complexity, useful for fatigue or cognitive-load studies.
Spatial Features
For multi-channel EEG, spatial filters such as Common Spatial Patterns (CSP) isolate task-specific cortical sources.
Stage 3: Classification & Machine Learning — Teaching Machines to Read the Body
After feature extraction, machine-learning models map those features to outcomes: focused vs fatigued, gesture A vs gesture B, normal vs arrhythmic.
- Classical ML: SVM, LDA, Random Forest , effective for curated features.
- Deep Learning: CNNs, LSTMs, Graph CNNs , learn directly from raw or minimally processed data.
- Transfer Learning: Improves cross-subject performance by adapting pretrained networks.
- Edge Inference: Deploying compact models (TinyML, quantized CNNs) on embedded hardware to achieve < 10 ms latency.
This is where raw physiology becomes actionable intelligence.
Interpreting Results — Making Sense of the Numbers
A robust pipeline delivers meaning, not just data:
- Detecting stress or fatigue for adaptive feedback.
- Translating EEG patterns into commands for prosthetics or interfaces.
- Monitoring ECG spectral shifts to flag early arrhythmias.
- Quantifying EMG coordination for rehabilitation or athletic optimization.
Performance hinges on accuracy, latency, robustness, and interpretability, especially when outcomes influence safety-critical systems.
Challenges and Future Directions
Technical: Inter-subject variability, electrode drift, real-world noise, and limited labeled datasets still constrain accuracy.
Ethical / Explainability: As algorithms mediate more decisions, transparency and consent are non-negotiable.
Multimodal Fusion: Combining EEG + EMG + ECG data improves reliability but raises synchronization and power-processing challenges.
Edge AI & Context Awareness: The next frontier is continuous, low-latency interpretation that adapts to user state and environment in real time.
Final Thought
Capturing a biosignal is only half the story. What truly powers next-gen neurotech and human-aware systems is turning that signal into sense. From electrodes and photodiodes to filters and neural nets, each link in this chain brings us closer to devices that don’t just measure humans; they understand them.

Every thought, heartbeat, and muscle twitch leaves behind a signal, but how do we actually capture them? In this blog post, we explore the sensors that make biosignal measurement possible, from EEG and ECG electrodes to optical and biochemical interfaces, and what it takes to turn those signals into meaningful data.
When we think of sensors, we often imagine cameras, microphones, or temperature gauges. But some of the most fascinating sensors aren’t designed to measure the world, they’re designed to measure you.
These are biosignal sensors: tiny, precise, and increasingly powerful tools that decode the electrical whispers of your brain, heart, and muscles. They're the hidden layer enabling brain-computer interfaces, wearables, neurofeedback systems, and next-gen health diagnostics.
But how do they actually work? And what makes one sensor better than another?
Let’s break it down, from scalp to circuit board.
First, a Quick Recap: What Are Biosignals?
Biosignals are the body’s internal signals, electrical, optical, or chemical , that reflect brain activity, heart function, muscle movement, and more. If you’ve read our earlier post on biosignal types, you’ll know they’re the raw material for everything from brain-computer interfaces to biometric wearables.
In this blog, we shift focus to the devices and sensors that make it possible to detect these signals in the real world, and what it takes to do it well.
The Devices That Listen In: Biosignal Sensor Types
.webp)
A Closer Look: How These Sensors Work
1. EEG / ECG / EMG – Electrical Sensors
These measure voltage fluctuations at the skin surface, caused by underlying bioelectric activity.
It’s like trying to hear a whisper in a thunderstorm; brain and muscle signals are tiny, and will get buried under noise unless the electrodes make solid contact and the amplifier filters aggressively.
There are two key electrode types:
- Wet electrodes: Use conductive gel or Saline for better signal quality. Still the gold standard in labs.
- Dry electrodes: More practical for wearables but prone to motion artifacts and noise (due to higher electrode resistance).
Signal acquisition often involves differential recording and requires high common-mode rejection ratios (CMRR) to suppress environmental noise.
Fun Fact: Even blinking your eyes generates an EMG signal that can overwhelm EEG data. That’s why artifact rejection algorithms are critical in EEG-based systems.
2. Optical Sensors (PPG, fNIRS)
These use light to infer blood flow or oxygenation levels:
- PPG: Emits light into the skin and measures reflection, pulsatile blood flow alters absorption.
- fNIRS: Uses near-infrared light to differentiate oxygenated vs. deoxygenated hemoglobin in the cortex.
Example: Emerging wearable fNIRS systems like Kernel Flow and OpenBCI Galea are making brain oxygenation measurement accessible outside labs.
3. Galvanic Skin Response / EDA – Emotion’s Electrical Signature
GSR (also called electrodermal activity) sensors detect subtle changes in skin conductance caused by sweat gland activity, a direct output of sympathetic nervous system arousal. When you're stressed or emotionally engaged, your skin becomes more conductive, and GSR sensors pick that up.
These sensors apply a small voltage across two points on the skin and track resistance over time. They're widely used in emotion tracking, stress monitoring, and psychological research due to their simplicity and responsiveness.
Together, these sensors form the foundation of modern biosignal acquisition — but capturing clean signals isn’t just about what you use, it’s about how you use it.
How Signal Quality Is Preserved
Measurement is just step one; capturing clean, interpretable signals involves:
- Analog Front End (AFE): Amplifies low signals while rejecting noise.
- ADC: Converts continuous analog signals into digital data.
- Signal Conditioning: Filters out drift, DC offset, 50/60Hz noise.
- Artifact Removal: Eye blinks, jaw clenches, muscle twitches.
Hardware platforms like TI’s ADS1299 and Analog Devices’ MAX30003 are commonly used in EEG and ECG acquisition systems.
New Frontiers in Biosignal Measurement
- Textile Sensors: Smart clothing with embedded electrodes for long-term monitoring.
- Biochemical Sensors: Detect metabolites like lactate, glucose, or cortisol in sweat or saliva.
- Multimodal Systems: Combining EEG + EMG + IMU + PPG in unified setups to boost accuracy.
A recent study involving transradial amputees demonstrated that combining EEG and EMG signals via a transfer learning model increased classification accuracy by 2.5–4.3% compared to EEG-only models.
Other multimodal fusion approaches, such as combining EMG and force myography (FMG), have shown classification improvements of over 10% compared to EMG alone.
Why Should You Care?
Because how we measure determines what we understand, and what we can build.
Whether it's a mental wellness wearable, a prosthetic limb that responds to thought, or a personalized neurofeedback app, it all begins with signal integrity. Bad data means bad decisions. Good signals? They unlock new frontiers.
Final Thought
We’re entering an era where technology doesn’t just respond to clicks, it responds to cognition, physiology, and intent.
Biosignal sensors are the bridge. Understanding them isn’t just for engineers; it’s essential for anyone shaping the future of human-aware tech.

In our previous blog, we explored how biosignals serve as the body's internal language—electrical, mechanical, and chemical messages that allow us to understand and interface with our physiology. Among these, electrical biosignals are particularly important for understanding how our nervous system, muscles, and heart function in real time. In this article, we’ll take a closer look at three of the most widely used electrical biosignals—EEG, ECG, and EMG—and their growing role in neurotechnology, diagnostics, performance tracking, and human-computer interaction. If you're new to the concept of biosignals, you might want to check out our introductory blog for a foundational overview.
"The body is a machine, and we must understand its currents if we are to understand its functions."-Émil du Bois-Reymond, pioneer in electrophysiology.
Life, though rare in the universe, leaves behind unmistakable footprints—biosignals. These signals not only confirm the presence of life but also narrate what a living being is doing, feeling, or thinking. As technology advances, we are learning to listen to these whispers of biology. Whether it’s improving health, enhancing performance, or building Brain-Computer Interfaces (BCIs), understanding biosignals is key.
Among the most studied biosignals are:
- Electroencephalogram (EEG) – from the brain
- Electrocardiogram (ECG) – from the heart
- Electromyogram (EMG) – from muscles
- Galvanic Skin Response (GSR) – from skin conductance
These signals are foundational for biosignal processing, real-time monitoring, and interfacing the human body with machines. In this article we look at some of these biosignals and some fascinating stories behind them.
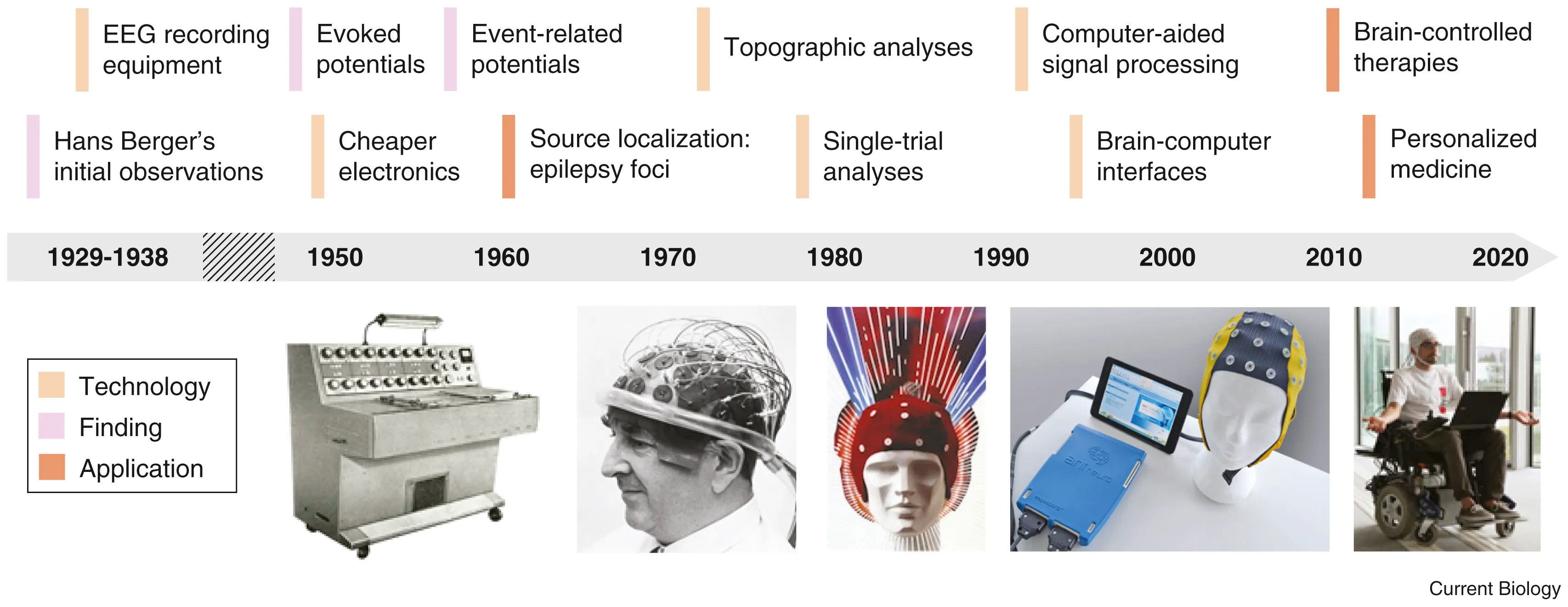
Electroencephalography (EEG): Listening to Brainwaves
In 1893, a 19 year old Hans Berger fell from a horse and had a near death experience. Little did he know that it would be a pivotal moment in the history of neurotechnology. The same day he received a telegram from his sister who was extremely concerned for him because she had a bad feeling. Hans Berger was convinced that this was due to the phenomenon of telepathy. After all, it was the age of radio waves, so why can’t there be “brain waves”? In his ensuing 30 year career telepathy was not established but in his pursuit, Berger became the first person to record brain waves.
When neurons fire together, they generate tiny electrical currents. These can be recorded using electrodes placed on the scalp (EEG), inside the skull (intracranial EEG), or directly on the brain (ElectroCorticogram). EEG signal processing is used not only to understand the brain’s rhythms but also in EEG-based BCI systems, allowing communication and control for people with paralysis. Event-Related Potentials (ERPs) and Local Field Potentials (LFPs) are specialized types of EEG signals that provide insights into how the brain responds to specific stimuli.
Electrocardiogram (ECG): The Rhythm of the Heart
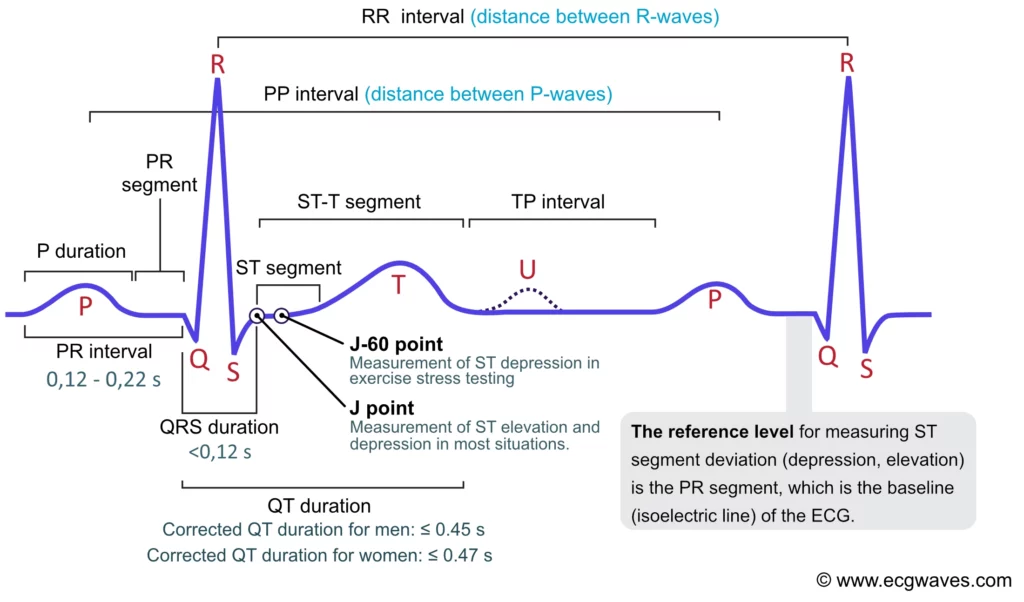
The heart has its own internal clock which produces tiny electrical signals every time it beats. Each heartbeat starts with a small electrical impulse made by a special part of the heart called the sinoatrial (SA) node. This impulse spreads through the heart muscle and makes it contract, first the upper (atria) and then lower chambers (ventricles) – that’s what pumps blood. This process produces voltage changes, which can be recorded via electrodes on the skin.
This gives rise to the classic PQRST waveform, with each component representing a specific part of the heart’s cycle. Modern wearables and medical devices use ECG signal analysis to monitor heart health in real time.
Fun fact: The waveform starts with “P” because Willem Einthoven left room for earlier letters—just in case future scientists discovered pre-P waves! So, thanks to a cautious scientist, we have the quirky naming system we still follow today.
Electromyography (EMG): The Language of Movement
When we perform any kind of movement - lifting our arm, kicking our leg, smiling, blinking or even breathing- our brain sends electrical signals to our muscles telling them to contract. When these neurons, known as motor neurons fire they release electrical impulses that travel to the muscle, causing it to contract. This electrical impulse—called a motor unit action potential (MUAP)—is what we see as an EMG signal. So, every time we move, we are generating an EMG signal!
Medical Applications
Medically, EMG is used for monitoring muscle fatigue especially in rehabilitation settings and muscle recovery post-injury or surgery. This helps clinicians measure progress and optimize therapy. EMG can distinguish between voluntary and involuntary movements, making it useful in diagnosing neuromuscular disorders, assessing stroke recovery, spinal cord injuries, and motor control dysfunctions.
Performance and Sports Science
In sports science, EMG can tell us muscle-activation timing and quantify force output of muscle groups. These are important factors to measure performance improvement in any sport. The number of motor units recruited and the synergy between muscle groups, helps us capture “mind-muscle connection” and muscle memory. Such things which were previously spoken off in a figurative manner can be scientifically measured and quantified using EMG. By tracking these parameters we get a window into movement efficiency and athletic performance. EMG is also used for biofeedback training, enabling individuals to consciously correct poor movement habits or retrain specific muscles
Beyond medicine and sports, EMG is used for gesture recognition in AR/VR and gaming, silent speech detection via facial EMG, and next-gen prosthetics and wearable exosuits that respond to the user’s muscle signals. EMG can be used in brain-computer interfaces (BCIs), helping paralyzed individuals control digital devices or communicate through subtle muscle activity. EMG bridges the gap between physiology, behavior, and technology—making it a critical tool in healthcare, performance optimization, and human-machine interaction.
As biosignal processing becomes more refined and neurotech devices more accessible, we are moving toward a world where our body speaks—and machines understand. Whether it’s detecting the subtlest brainwaves, tracking a racing heart, or interpreting muscle commands, biosignals are becoming the foundation of the next digital revolution. One where technology doesn’t just respond, but understands.

